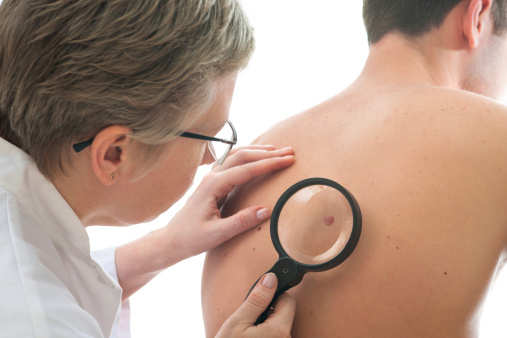
גיל מבוגר ומין זכר מלווים בסיכון מוגבר לאבחנה של מלנומה ראשונית נוספת (JAMA Dermatol)
לאחר אבחנה ראשונה של מלנומה פולשנית ראשונית, גיל מבוגר ומגדר זכרי הינם גורמי סיכון אפשריים לאבחנה של מלנומה נוספת בשלב

לאחר אבחנה ראשונה של מלנומה פולשנית ראשונית, גיל מבוגר ומגדר זכרי הינם גורמי סיכון אפשריים לאבחנה של מלנומה נוספת בשלב

אימון שרירי רצפת אגן מלווה בשיפור בתפקוד המיני בנשים עם הפרעה בתפקוד רצפת האגן, כולל שיפור עוררות מינית, אורגזמה, שביעות

במאמר שפורסם בכתב העת International Journal of Cancer מדווחים חוקרים על תוצאות מחקר חדש, מהן עולה כי שתיית קפה עשויה

איזוטרטינואין (רואקוטן) הינה נגזרת של רטינואידים (ויטמין A) המשמשת לטיפול באקנה וולגריס (Acne Vulgaris) קשה שלא הגיבה לקוי טיפול מוקדמים. ד”ר עדי זיו, עורך מדור ילדים, משתף מחקר מה-JAMA Dermatology שעסק בהערכת הסיכון היחסי והמוחלט לאובדנות ולתחלואה נפשית בקרב המשתמשים באיזוטרטינואין, ומוסיף מהערותיו.

פרופ’ רז עוסק בהפסקת/לקיחת אספירין במטופלים, ומתייחס למאמר שפורסם לאחרונה ב-JAMA בו נמצא שבמטופלים ללא אינדיקציה לנטילת אספירין, הפסקת הטיפול
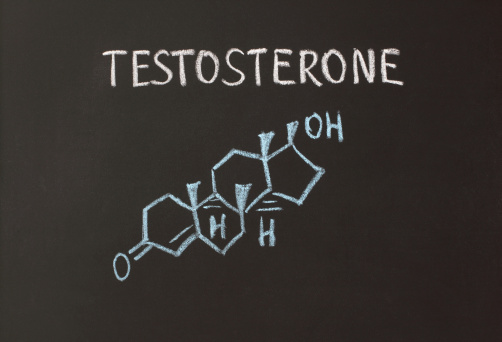
עדויות חדשות תומכות בבטיחות טיפול חליפי בטסטוסטרון בגברים בגיל העמידה וגברים מבוגרים יותר עם היפוגונאדיזם, כאשר אין עדות לעליה בסיכון
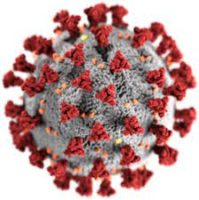
בנשים לאחר טיפולי IVF/ICSI הדבקה בנגיף קורונה בתוך עשרה שבועות מהחזרת עוברים אינה משפיעה לרעה על תוצאות היריון, שיעורי השרשה

במאמר שפורסם בכתב העת International Journal of Oncology מדווחים חוקרים על תוצאות מחקר חדש, מהן עולה כי חשיפה תעסוקתית לאזבסט

למרות חשש מפני תביעות רשלנות רפואית של רופאים, חוקרים לא מצאו עליה בתביעות רשלנות רפואית מוצלחות על-רקע מעקב פעיל כגישת
במאמר שפורסם בכתב העת Journal of the American College of Cardiology מדווחים חוקרים על תוצאות מחקר חדש,
בחולים עם אבחנה של אנגיואדמה תורשתית תועדה ירידה בשיעור ההתקפים החודשי עם טיפול ב- Lanadelumab, כך עולה

במאמר שפורסם בכתב העת JAMA מדווחים חוקרים על תוצאות מחקר חדש, מהן עולה כי בפגים עם בקע
בשל האיומים האחרונים, משרד הבריאות מפרסם את מספרי הטלפון של מרכזי החוסן ומרכזי החירום והתמיכה הרגשית של קופות החולים.
טלפון מרכז החוסן הארצי הטיפולי *5486 – המרכז פועל בימים א-ה בין השעות 08:00-20:00
קופות חולים: מאוחדת 3833 – לאומית 507* – מכבי 3555* – כללית 8703 *
במאמר שפורסם בכתב העת Cureus מדווחים חוקרים על תוצאות מחקר חדש, מהן עולה כי רמות CRP (או
בהשוואה לטיפול כימותרפי, טיפול כימו-אימונותרפי קדם-ניתוחי הוביל לשיעורי הישרדות ללא-אירועים טובים יותר ושיעורי תגובה מלאה גבוהים יותר,

במאמר שפורסם בכתב העת New England Journal of Medicine מדווחים חוקרים על תוצאות מחקר חדש בו הגיוס למחקר בשלב מוקדם בשל סוגיות הנוגעות לבטיחות ההתערבות, כאשר חיסון אימהי כנגד RSV המבוסס על Perfusion F Protein אמנם הפחית סיכון לסיבוכים משנית למחלה בדרכי נשימה תחתונות על-רקע RSV, אך לווה בעליה בסיכון ללידה מוקדמת. ד”ר ברנרד ברזילי, עורך מדור ניאונטולוגיה, משתף את המאמר ומוסיף מהערותיו

איזוטרטינואין (רואקוטן) הינה נגזרת של רטינואידים (ויטמין A) המשמשת לטיפול באקנה וולגריס (Acne Vulgaris) קשה שלא הגיבה לקוי טיפול מוקדמים. ד”ר עדי זיו, עורך מדור ילדים, משתף מחקר מה-JAMA Dermatology שעסק בהערכת הסיכון היחסי והמוחלט לאובדנות ולתחלואה נפשית בקרב המשתמשים באיזוטרטינואין, ומוסיף מהערותיו.

במרכזו של סיפור מקרה זה מתוך ה-JAMA גבר בן 69 שהגיע למרפאה הרֵאומטולוגית 3 שבועות לאחר שאושפז עקב חום, עייפות, פריחה, בצקת היקפית מצד ימין וקוצר נשימה.

בקיצור נמרץ – לקט מחקרים מן המחצית השניה של חודש מארס 2024

בגליון זה: טכיקרדיה על-חדרית, הטיפול בחסר ויטמין D בילדים, בקיצור נמרץ ועוד

במגזין זה: קוצבי לב ודפיברילטורים מושתלים, ניוון מקולרי גילי, בקיצור נמרץ ועוד

מחקר זה מתעמק באתגרים העומדים לפני צעירים הסובלים מאסתמה, תוך דגש על ההיענות לקורטיקוסטרואידים בשאיפה (ICS), טיפול מרכזי באסתמה מתמשכת.

גולש יקר ! ידיעה זו מיועדת לסגל הרפואי בלבד ואינה מאושרת לצפייה לקהל הרחב. אנא בצע LOG

ב-1 בנובמבר התקיים וובינר שעסק בניהול טראומה ראשונית ונפשית, במסגרת מיזם “איגודים נפגשים” של האיגוד הישראלי לרפואת המשפחה, שאירח את

לפניכם הרצאות קצרות על עדכונים באימונותרפיה לטיפול בסרטן עם פרופ’ גל מרקל. בחסות בלתי תלויה של

לפניכם הרצאה ודיון שנערכו במסגרת קצר קולע וממוקד – סוגיות בתוספי תזונה ההרצאה בחסות חברת אלטמן

הנכם מוזמנים לצפות בתיעוד הכנס הכולל את ההרצאות וספר התקצירים באתר החדש של החברה להשתלות

לפניכם הרצאתו של פרופ’ יוחאי אדיר, יו”ר האיגוד הישראלי לרפואת ריאות, מנהל מכון הריאה מרכז רפואי כרמל, מתוך הכנס השנתי
בקליפ הקצרצר שלהלן מוצג מקרה מייצג חשוב ומעניין המדגים את היעילות של שילוב אופדיבו, יירבוי וכימו
הרצאה ודיון עם פרופ’ אבישי אליס ופרופ’ צבי פרידלנדר במסגרת תכנית הוובינרים של האיגוד לרפואה פנימית לכניסה

צפו בהרצאתו של פרופ’ גבריאל איזביצקי, מומחה ברפואה פנימית וריאות, מנהל מכון ריאות במרכז רפואי שערי צדק,

מנהל בית החולים ‘אסותא’ אשדוד, ד”ר ארז ברנבוים, מנהל המרכז הרפואי האוניברסיטאי ברזילי, ד”ר חזי לוי, ומנהל המרכז הרפואי האוניברסיטאי סורוקה, ד”ר שלומי קודש יקבלו השנה את ‘פרס נשיא האוניברסיטה’ במסגרת חבר הנאמנים של אוניברסיטת בן-גוריון בנגב.

פרופ’ עודד נחליאלי, מנהל היחידה לכירורגיית הפה, הלסתות ובלוטות הרוק בא.ר.ם, המרכז הרב-תחומי לרפואת אף אוזן גרון ומנהל היחידה לכירורגיה פולשנית מזערית של הפה, הפנים והלסתות במרכז הרפואי לגליל, בנהריה, מונה לאחרונה על ידי החברה האמריקאית והבינלאומית לכירורגיית אף אוזן גרון וניתוחי ראש צוואר לחבר בוועדה לקביעת קריטריונים בינלאומיים, להתמחות בתחום האנדוסקופיות והכירורגיה המזערית של בלוטות הרוק.

המוקד, המופעל ממטעם ארגון הרוקחות בישראל בשיתוף האגודה לזכויות החולה בישראל ייפתח מחדש השבוע במתכונתו המחודשת. המוקד יתן מענה מקצועי לא רק לשאלות מצד הקהל הרחב, אלא גם לשאלות קליניות מצד צוותי הרפואה והפרא-רפואה. מספר טלפון מוקד הייעוץ הרוקחי-קליני מטעם ארגון הרוקחות בישראל כוכבית 9711 *. המוקד פעיל בימים א’, ג’, ה’ בין השעות 10:00 – 13:00, ובימים ב’ ו-ד’ בין השעות 16:00 – 19:00. המוקד זמין בשפות עברית, אנגלית, ערבית ורוסית.

לקראת חג הפסח משרד הבריאות בשיתוף המרכז הארצי למידע בהרעלות במרכז רפואי ברמב”ם וארגון בטרם לבטיחות ילדים, מפרסמים נתונים על הרעלות בעקבות חומרי ניקיון וכללים לבטיחות השימוש בחומרי ניקיון בבית.

אתמול (ג’, 5.3.24) נכנס לתוקפו עדכון תקנות מעברים של מבוטחים בין קופות החולים. בעדכון התקנות מוארך המועד בו ניתן לבטל את המעבר בין קופת חולים אחת לשנייה, מ-45 ימים לפני כניסת המעבר לתוקף ל-10 ימים לפני כניסת המעבר לתוקף.

האיגוד הנוירולוגי בשילוב העמותה הישראלית למדעי המוח ועמותות החולים מזמינים את הקבל הרחבליריד המוח-נפלאות המוח בבריאות ובחולי. יריד ערב אשר

יחידת הריאות במרכז הרפואי זיו בניהולה של ד”ר ענת עמיטל, מקיימת ערב לרופאים, יום ד’ ה- 10 בינואר, בשעה 18:30.

ירחי כלה הבינלאומי ה- 20 ביד שרה. לטפל בהורים קשישים זוהי זכות, לראשונה נשאל את השאלות הכי

לתוכנית המלאה לחצו כאן בבקשה

על רקע הדיון המחודש בנושא מעורר המחלוקת ייערך כנס רחב היקף במכללה האקדמית גליל מערבי בהשתתפות ח”כים,
נא ציין כתובת דואר אלקטרוני שבה אתה מקבל דיוורי e-Med או זו שציינת בכרטיס האישי שלך.
קישור לאיפוס סיסמא נשלח למייל.
חזרה להתחברות
תגובות אחרונות